PATIENT INSTRUCTION GUIDES
The following symbols may appear on your ACUVUE® Brand Contact Lens package.
| Symbol | Title | Description | Reference # |
|---|---|---|---|
 |
Manufacturer | Indicates the medical device manufacturer | 5.1.1 |
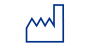 |
Date of Manufacture | Indicates the date when the medical device was manufactured | 4.1.3 |
 |
Use-by Date | Indicates the date after which the medical device is not to be used | 4.1.3 |
 |
Batch Code | Indicates the manufacturer's batch code so that the batch or lot can be identified | 5.2.5 |
 |
Sterilized Using Steam Heat | Indicates a medical device has been sterilized using steam heat | 5.4.2 |
 |
Do Not Reuse | Indicates a medical device that is intended for one use | 5.4.3 |
 |
Consult Instructions for Use | Indicates the need for the user to consult the instructions for use | 5.4.3 |
The information presented in this glossary is from ISO 15223-1: 2016 MedicalDevices - Symbols to be used with medical labels, labeling and information to be supplied - Part 1: General requirements.
Revision Date: December 2016
Get official instructional materials for all ACUVUE® Brand Contact Lenses right here.
ACUVUE® OASYS® Brand 2-Week with HYDRACLEAR® PLUS
ACUVUE OASYS® Brand for ASTIGMATISM
ACUVUE OASYS® 1-Day with HydraLuxe™
ACUVUE OASYS® 1-Day with HydraLuxe™ for ASTIGMATISM
1-DAY ACUVUE® MOIST Brand
1-DAY ACUVUE® MOIST Brand for ASTIGMATISM
1-DAY ACUVUE® MOIST Brand MULTIFOCAL
1-DAY ACUVUE® DEFINE® Brand with LACREON® Technology
1-MONTH ACUVUE® VITA™ with HYDRAMAX™ Technology
1-MONTH ACUVUE® VITA™ with HYDRAMAX™ Technology for ASTIGMATISM
Patient Instruction Guide (520 KB)